

Liver cancer is one of the most common types of cancer. The mortality rate of liver cancer is very high and the 5-year survival rate is extremely low, mainly because the majority of patients are diagnosed at an advanced stage. There are two main reasons for this: first, patients’ poor compliance and failure to strictly follow the protocols recommended by the guidelines for liver cancer surveillance. At present, for those who are at high risk of liver cancer as stipulated by the guidelines, routine screening is recommended every 6 months. On the other hand, the sensitivity of AFP test and ultrasonography, which are currently used in our clinic, can only reach 60-70%, and there is a serious lack of highly sensitive and specific markers for screening and diagnosis of early stage HCC. cfDNA liquid biopsy liver cancer early screening and diagnosis technology provides a possible solution to this painful problem.
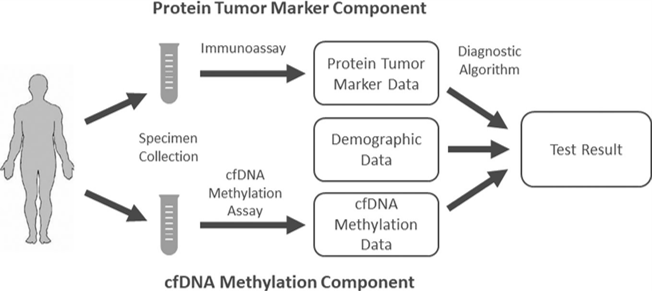
Helio Liver Test, a blood test that combines cfDNA methylation patterns, clinical variables, and tumor protein markers for comprehensive analysis. A randomized, double-blind, multicenter prospective clinical trial was conducted to validate the performance of HelioLiver Test. The final data showed that HelioLiver Test has a sensitivity of 85% for detecting liver cancer, 76% for early stage (AJCC stage I and II), as well as a specificity of 91%, which is significantly better than AFP and GALAD models.


